Anticancer Drugs Market Set to Expand at 9.14% CAGR, Reaching USD 401.32 Billion by 2034
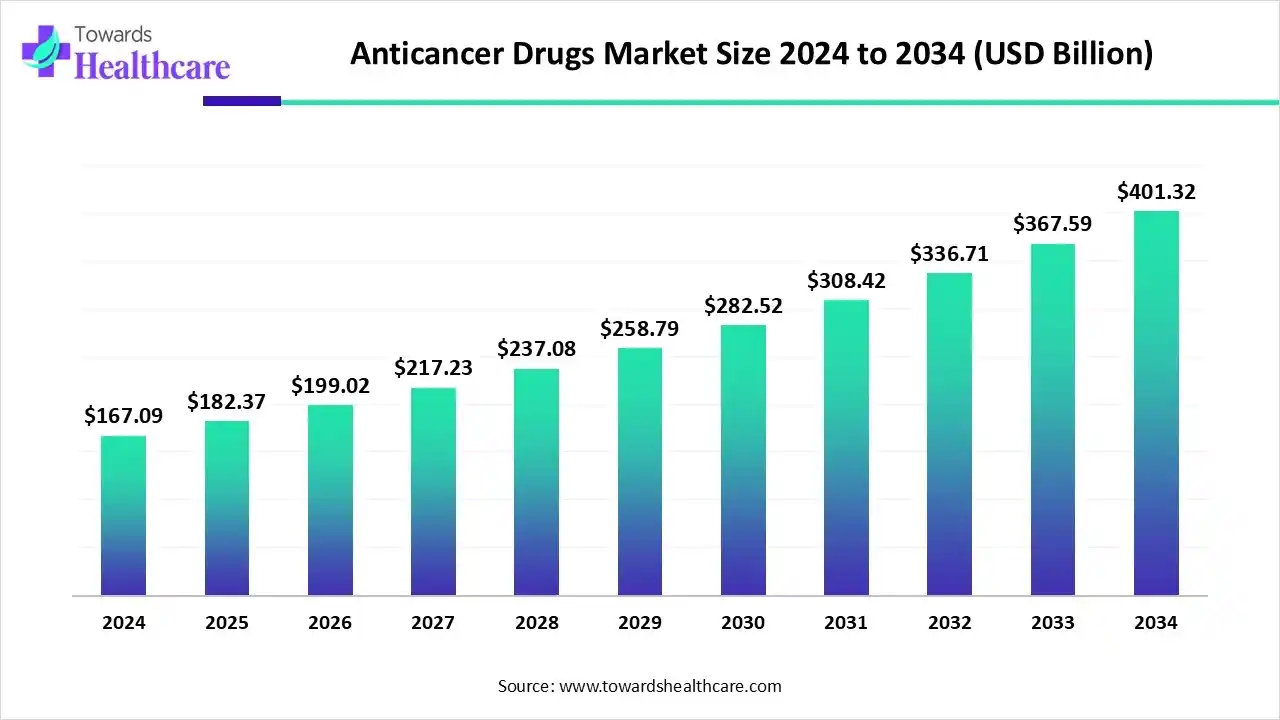
The global anticancer drugs market size is calculated at USD 182.37 billion in 2025 and is expected to reach around USD 401.32 billion by 2034, growing at a CAGR of 9.14% for the forecasted period.
Ottawa, Nov. 21, 2025 (GLOBE NEWSWIRE) -- The global anticancer drugs market size was valued at USD 167.09 billion in 2024 and is predicted to hit around USD 401.32 billion by 2034, rising at a 9.14% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
The growth is driven by the increasing incidence of cancer, advancements in targeted therapies like immunotherapies and monoclonal antibodies, and government support for R&D.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6322
Key Takeaways
- North America held a major revenue share of approximately 40% in the anticancer drugs market in 2024.
- Asia-Pacific is expected to witness the fastest growth during the predicted timeframe.
- By drug class/modality, the chemotherapy segment registered its dominance over the global market with a share of approximately 35% in 2024.
- By Drug class/modality, the targeted therapy segment is expected to grow with the highest CAGR in the market during the studied years.
- By route of administration, the intravenous (IV) segment held the largest revenue share of approximately 55% in the market in 2024.
- By route of administration, the oral segment is expected to show the fastest growth over the forecast period.
- By cancer type/indication, the breast cancer segment held a dominant presence in the anticancer drugs market with a share of approximately 22% in 2024.
- By cancer type/indication, the haematological cancers segment is expected to witness the fastest growth in the market over the forecast period.
Market Overview & Potential
The anticancer drugs market is mainly driven by the increasing rate of cancers, the growing elderly population, and the rising need for advanced treatments that boost survival rates and quality of life. Anti-cancer drugs also called oncology drugs, are medicines designed to prevent, inhibit, or treat cancer by targeting fast-dividing cells or specific molecular pathways involved in tumour growth. They function by destroying cancer cells, blocking growth signals, or boosting the immune system’s response. Ongoing research, technological advances, and personalised medicine are broadening the effectiveness and use of these drugs worldwide.
Quick Facts Table
| Table | Scope | |
| Market Size in 2025 | USD 182.37 Billion | |
| Projected Market Size in 2034 | USD 401.32 Billion | |
| CAGR (2025 - 2034) | 9.14 | % |
| Leading Region | North America | |
| Market Segmentation | By Drug Class / Modality, By Route of Administration, By Cancer Type / Indication, By Region | |
| Top Key Players | Roche/Genentech, Novartis, Pfizer, Merck & Co. (MSD), Bristol Myers Squibb (BMS), AstraZeneca, Johnson & Johnson/Janssen, Sanofi, Amgen, Eli Lilly & Co., Takeda, BeiGene, Regeneron, Bluebird Bio, Seagen, Moderna, Genmab, Incyte, Exelixis, Hutchmed / Chi-Med | |
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What is the Growth Potential Responsible for The Growth of The Anticancer Drugs Market?
The main drivers of the anticancer drugs market include the rising global incidence of cancer, advancements in R&D leading to new therapies like targeted therapies and immunotherapy, and an ageing global population. Other factors include increased cancer awareness and screening, improved healthcare access and infrastructure, government initiatives, and a growing demand for personalised and combination therapies.
What Are the Growing Trends Associated with the Anticancer Drugs Market?
Growth of immunotherapy: Immunotherapy, including checkpoint inhibitors like PD-1/PD-L1, is becoming a mainstream treatment option across various solid tumours due to its strong efficacy.
Increasing importance of biosimilars: Biosimilars are growing in popularity, especially in emerging markets, because they offer a lower-cost alternative to expensive original biologic drugs, making treatment more accessible.
Focus on personalised medicine: Advances in diagnostics and research are enabling oncologists to tailor treatments based on a patient's specific cancer profile, leading to better outcomes and driving demand for personalised drugs.
Market expansion and partnerships: The market is expanding globally, with significant growth expected in both developed and emerging economies. Pharmaceutical companies are also actively engaging in strategic partnerships and collaborations to accelerate drug discovery and development.
Development of novel drugs: Companies are investing heavily in R&D for new drugs with different mechanisms of action, which is a major opportunity for market growth. This includes research into areas like targeted protein degraders and radiopharmaceuticals.
What Is the Growing Challenge in the Anticancer Drugs Market?
Challenges in the anticancer drugs market include high research and development (R&D) costs, long development times, and low success rates. Other major obstacles are the high cost of drugs leading to reimbursement and access issues, the development of drug resistance, and biological complexities like tumour heterogeneity. Regulatory, supply chain, and logistical issues, such as drug shortages, also create significant challenges for the market.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Clinical Trial for Anticancer Drugs
| Trial ID | Therapy | Cancer Type | Objective | Status |
| NCT05257408 | Relacorilant | Ovarian Cancer | Evaluate efficacy in platinum-resistant cases | NDA submitted |
| NCT05257408 | Decitabine + Venetoclax | Acute Myeloid Leukemia | Assess safety and efficacy in newly diagnosed patients ineligible for intensive chemotherapy. | NDA accepted |
| NCT05257408 | Pembrolizumab | Head and Neck Cancer | Evaluate as perioperative therapy | FDA approved |
| NCT05257408 | Encora + Cetuximab | Colorectal Cancer | Assess efficacy in BRAF-mutated metastatic cases | FDA approved |
| NCT05257408 | Niraparib | Prostate Cancer | Evaluate in combination with hormone therapy | Phase 3 completed |
| NCT05257408 | Zepzelca + Tecentriq | Small Cell Lung Cancer | Assess as first-line maintenance therapy | FDA approved |
| NCT05257408 | Sonrotoclax | Blood Cancer | Evaluate as an alternative to Venclexta | Regulatory review ongoing |
| NCT05257408 | BGB-43395 | Breast Cancer | Evaluate as CDK4-specific therapy | Early-phase trial |
| NCT05257408 | GLSI-100 | HER2-Positive Breast Cancer | Evaluate patients with the HLA-A*02 genotype | Fast track designation |
| NCT05257408 | Tabelecleucel | EBV+ PTLD | Assess efficacy in Phase 3 trial | FDA resubmission |
| NCT05257408 | Avmapki Fakzynja Co-Pack | Ovarian Cancer | Evaluate in KRAS-mutated recurrent cases | Approved |
| NCT05257408 | Imaavynipocalimab-aahu | Myasthenia Gravis | Assess efficacy | Approved |
| NCT05257408 | Relacorilant | Ovarian Cancer | Evaluate in platinum-resistant cases | NDA submitted |
| NCT05257408 | Decitabine + Venetoclax | Acute Myeloid Leukemia | Assess safety and efficacy in newly diagnosed patients ineligible for intensive chemotherapy. | NDA accepted |
| NCT05257408 | Pembrolizumab | Head and Neck Cancer | Evaluate as perioperative therapy | FDA approved |
| NCT05257408 | Encora + Cetuximab | Colorectal Cancer | Assess efficacy in BRAF-mutated metastatic cases | FDA approved |
| NCT05257408 | Niraparib | Prostate Cancer | Evaluate in combination with hormone therapy | Phase 3 completed |
| NCT05257408 | Zepzelca + Tecentriq | Small Cell Lung Cancer | Assess as first-line maintenance therapy | FDA approved |
| NCT05257408 | Sonrotoclax | Blood Cancer | Evaluate as an alternative to Venclexta | Regulatory review ongoing |
| NCT05257408 | BGB-43395 | Breast Cancer | Evaluate as CDK4-specific therapy | Early-phase trial |
| NCT05257408 | GLSI-100 | HER2-Positive Breast Cancer | Evaluate patients with the HLA-A*02 genotype | Fast track designation |
| NCT05257408 | Tabelecleucel | EBV+ PTLD | Assess efficacy in Phase 3 trial | FDA resubmission |
| NCT05257408 | Avmapki Fakzynja Co-Pack | Ovarian Cancer | Evaluate in KRAS-mutated recurrent cases | Approved |
| NCT05257408 | Imaavynipocalimab-aahu | Myasthenia Gravis | Assess efficacy | Approved |
Regional Analysis
How Did North America Dominate the Anticancer Drugs Market In 2024?
North America held a major revenue share of approximately 40% in the anticancer drugs market in 2024. The North American anticancer drugs market is a large and growing sector, driven by the increasing incidence of cancer and a rise in demand for advanced therapies like targeted treatments and immunotherapies. Key factors influencing market expansion include advancements in drug discovery, the increasing prevalence of various cancer types, and the role of generic and biosimilar drugs in providing more affordable treatment options.
What Made the Asia Pacific Significantly Grow in The Anticancer Drugs Market In 2024?
Asia-Pacific is expected to witness the fastest growth during the predicted timeframe. The Asia Pacific (APAC) anticancer drugs market is rapidly expanding, driven by a high cancer prevalence, a large population, and improving healthcare infrastructure. Key factors fueling growth include advancements in targeted and immunotherapy treatments, increased awareness and government initiatives for early diagnosis, and a rise in investments in pharmaceutical research and development in countries like China and Japan.
Segmental Insights
By drug class/modality,
The chemotherapy segment registered its dominance over the global market with a share of approximately 35% in 2024. Chemotherapy remains one of the most widely used cancer treatment modalities, employing cytotoxic agents that target rapidly dividing cells. Despite the rise of targeted and immunotherapies, chemotherapy continues to be essential in early-stage cancers, combination regimens, and haematological malignancies. Its affordability, wide applicability, and established clinical protocols maintain its strong market relevance across global oncology settings.
The targeted therapy segment is expected to grow with the highest CAGR in the market during the studied years. Targeted therapies act on specific molecular pathways, improving treatment outcomes with fewer adverse effects compared to traditional chemotherapy. This segment includes kinase inhibitors, monoclonal antibodies, and precision-based therapies driven by genomic profiling. Rapid adoption is supported by rising personalised medicine trends, expanding biomarker-driven drug development, and strong clinical success in breast cancer, lung cancer, and blood cancers.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By route of administration,
The intravenous (IV) segment held the largest revenue share of approximately 55% in the market in 2024. Intravenous administration is the dominant route for hospital-based oncology treatments due to its high bioavailability and rapid therapeutic action. It is preferred for potent chemotherapeutics, monoclonal antibodies, and combination regimens. Increasing cancer treatment centres, infusion clinics, and complex biologic therapies continue to support the demand for IV anticancer drug delivery.
The oral segment is expected to show the fastest growth over the forecast period. Oral anticancer drugs are increasingly adopted for patient convenience, reduced hospital visits, and improved quality of life. This segment includes targeted therapies, hormone therapies, and certain chemotherapies formulated for long-term outpatient use. The growing development of orally available kinase inhibitors and rising preference for at-home cancer management continue to strengthen this segment.
By cancer type/indication,
The breast cancer segment held a dominant presence in the anticancer drugs market with a share of approximately 22% in 2024. Breast cancer is one of the largest oncology drug segments, driven by high global prevalence and expanding use of targeted therapies such as HER2 inhibitors and CDK4/6 inhibitors. Continuous innovation, including antibody drug conjugates (ADCs) and personalised treatment approaches, maintains strong market growth. Screening initiatives and earlier diagnosis also accelerate drug uptake.
The haematological cancers segment is expected to witness the fastest growth in the market over the forecast period. Haematological cancers, including leukaemia, lymphoma, and multiple myeloma, represent a rapidly advancing area of oncology, driven by monoclonal antibodies, CAR-T therapies, and next-generation targeted drugs. Strong clinical outcomes, high relapse rates requiring continuous therapy, and expanding cell and gene therapy pipelines support sustained market demand. Precision-based treatment strategies further accelerate segment growth.
Browse More Insights of Towards Healthcare:
The cancer biomarkers market size stood at US$ 38.62 billion in 2025, grew to US$ 43.54 billion in 2026, and is forecast to reach US$ 128 billion by 2035, expanding at a CAGR of 12.73% from 2026 to 2035.
The global cancer supportive care drugs market size is calculated at US$ 21.89 billion in 2024, grew to US$ 22.36 billion in 2025, and is projected to reach around US$ 27.05 billion by 2034. The market is expanding at a CAGR of 2.14% between 2025 and 2034.
The global cancer diagnostics market size is calculated at USD 109.65 billion in 2024, grow to USD 116.42 billion in 2025, and is projected to reach around USD 199.54 billion by 2034, rising at a 6.17% CAGR for the forecasted period of 2025 to 2034.
The global cancer stem cells market size is calculated at US$ 3.2 in 2024, grew to US$ 3.51 billion in 2025, and is projected to reach around US$ 8.04 billion by 2034. The market is expanding at a CAGR of 9.64% between 2025 and 2034.
The global cancer biologics market size is expected to increase from USD 119.41 billion in 2025 to USD 232.02 billion by 2034, growing at a CAGR of 7.66% throughout the forecast period from 2025 to 2034.
The cancer vaccines market size is anticipated to grow from USD 11.38 billion in 2025 to USD 33.61 billion by 2034, with a compound annual growth rate (CAGR) of 11.44% during the forecast period from 2025 to 2034.
The worldwide cancer drug manufacturing market is experiencing significant expansion, with projections indicating a revenue increase reaching several hundred million dollars by the end of the forecast period, spanning 2025 to 2034.
The global cancer biopsy market size is calculated at US$ 30.81 billion in 2024, grew to US$ 36.61 billion in 2025, and is projected to reach around US$ 163.29 billion by 2034. The market is expanding at a CAGR of 18.84% between 2025 and 2034.
The global AI in cancer diagnostics market size is calculated at USD 1.07 billion in 2024 and is expected to be worth USD 2.61 billion by 2034, expanding at a CAGR of 9.35% from 2024 to 2034.
The worldwide lung cancer trial market is experiencing significant expansion, with projections indicating a revenue increase reaching several hundred million dollars by the end of the forecast period, spanning 2025 to 2034.
Recent Developments
- On October 2, 2025, the U.S.FDA approved the combination of lurbinectedin (Zepzelca) and atezolizumab (Tecentriq) for maintenance treatment in adults with extensive-stage small cell lung cancer (ES-SCLC) whose disease has not progressed after first-line induction therapy. This combination aims to improve survival outcomes in this patient group.
- In October 2025, AstraZeneca and Daiichi Sankyo announced that their precision drug, Datroway, improved survival prospects in patients with advanced breast cancer in a late-stage trial. This development paves the way for broader approvals and potential market expansion.
- On September 19, 2025, the U.S. FDA approved pembrolizumab and berahyaluronidase alfa-pmph (Keytruda Qlex) for subcutaneous injection in adult and pediatric patients aged 12 years and older with solid tumour indications previously treated with intravenous pembrolizumab. This approval offers a more convenient administration option for patients.
- In September 2025, AbbVie launched its ovarian cancer drug, Elahere, in the UK at a list price matching that in the U.S. This strategic move aims to expand the drug's availability and accessibility to patients in the UK.
Anticancer Drugs Market Key Players List
- Roche/Genentech - biologics, targeted therapies, mAbs
- Novartis - targeted therapies, kinase inhibitors, CAR-T
- Pfizer - small-molecule and immunotherapy
- Merck & Co. (MSD) - checkpoint inhibitors, vaccines
- Bristol Myers Squibb (BMS) - immunotherapies, CAR-T
- AstraZeneca - targeted therapies, antibody-drug conjugates
- Johnson & Johnson/Janssen - hematologic and solid tumour therapies
- Sanofi - biologics and combination cancer therapies
- Amgen - biologics, targeted therapies, biosimilars
- Eli Lilly & Co. - small-molecule oncology drugs
- Takeda - targeted therapies and haematology
- BeiGene - innovative oncology biologics and immunotherapy
- Regeneron - monoclonal antibodies
- Bluebird Bio - cell & gene therapy
- Seagen - antibody-drug conjugates
- Moderna - mRNA-based cancer vaccines
- Genmab - monoclonal antibody development
- Incyte - kinase inhibitors and immuno-oncology
- Exelixis - small-molecule oncology
- Hutchmed / Chi-Med - China-based oncology therapeutics
Value Chain Analysis
R&D
R&D in anti-cancer drugs begins with target identification and compound screening, followed by preclinical testing to evaluate safety and efficacy. Formulation development and optimization prepare promising compounds for clinical trials. Key organizations involved include Roche, Novartis, Pfizer, AstraZeneca, and Bristol-Myers Squibb.
Clinical Trials & Approval
Clinical trials start with Phase I to assess safety, Phase II to determine efficacy, and Phase III for large-scale validation. Successful trials lead to regulatory submission and approval. Major organizations include the National Cancer Institute (NCI), the American Cancer Society (ACS), Roche, Novartis, and Pfizer.
Patient Support and Service
Patient support involves awareness programs, counseling, and financial assistance, followed by adherence monitoring and post-treatment care to improve outcomes. Key organizations providing these services include CancerCare, American Cancer Society, local hospital networks, and oncology clinics.
Top Vendors in Anticancer Drugs Market & Their Offerings:
Roche
Key Offerings:
- Herceptin – Targets HER2-positive breast cancer.
- Avastin – Inhibits angiogenesis in various cancers.
- Tecentriq – PD-L1 inhibitor for multiple cancer types.
- Recent Developments: In 2025, Roche's anti-cancer drug Atezolizumab received CDSCO panel approval for metastatic triple-negative breast cancer in India.
Novartis
Key Offerings:
- Kymriah – CAR-T cell therapy for certain leukemias.
- Kisqali – CDK4/6 inhibitor for HR-positive breast cancer.
- Recent Developments: In 2025, Novartis announced the launch of Kisqali Plus, an enhanced formulation of Kisqali, showing improved efficacy and reduced side effects in early-stage breast cancer patients.
Pfizer
Key Offerings:
- Ibrance – CDK4/6 inhibitor for HR-positive breast cancer.
- Xalkori – ALK inhibitor for non-small cell lung cancer (NSCLC).
- Recent Developments: In 2025, Pfizer received FDA approval for Xalkori XR, an extended-release formulation offering improved patient compliance in NSCLC treatment.
Bristol-Myers Squibb (BMS)
Key Offerings:
- Opdivo – PD-1 inhibitor for various cancers.
- Yervoy – CTLA-4 inhibitor, often used in combination with Opdivo.
- Recent Developments: In 2025, BMS entered into an $11 billion partnership with BioNTech to co-develop BNT327, a bispecific antibody targeting two cancer cell receptors simultaneously, currently undergoing over 20 clinical trials.
Merck & Co.
Key Offerings:
- Keytruda – PD-1 inhibitor for various cancers.
- Lynparza – PARP inhibitor for ovarian and breast cancers.
- Recent Developments: In 2025, Merck received FDA approval for Keytruda Qlex, a subcutaneous formulation of Keytruda, offering patients a more convenient administration option.
AstraZeneca
Key Offerings:
- Tagrisso – EGFR inhibitor for NSCLC.
- Lynparza – PARP inhibitor for ovarian and breast cancers.
- Recent Developments: In 2025, AstraZeneca's antibody-drug conjugate Datroway showed significant improvement in overall survival in a late-stage breast cancer trial, leading to expectations of broader regulatory approvals.
BeOne Medicines (formerly BeiGene)
Key Offerings:
- Brukinsa – BTK inhibitor for hematologic cancers.
- Sonrotoclax – BCL-2 inhibitor under development.
- Recent Developments: In 2025, BeOne's Brukinsa outperformed competitors with $950 million in sales in Q2, and its BCL-2 inhibitor, sonrotoclax, is under regulatory review in China, with potential U.S. approval by 2027.
Segments Covered in The Report
By Drug Class / Modality
- Chemotherapy
- Targeted Therapy
- Monoclonal Antibodies / Biologics
- Immunotherapy (Checkpoint inhibitors, CAR-T, TCR therapies)
- Hormone Therapy
- Others (Oncolytic viruses, combination therapies, adjuncts)
By Route of Administration
- Intravenous (IV)
- Oral
- Subcutaneous
- Topical / Localised
By Cancer Type / Indication
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Hematological Cancers (Leukemia, Lymphoma, Myeloma)
- Prostate Cancer
- Melanoma & Skin Cancers
- Other Cancers (Ovarian, Gastric, Liver, etc.)
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6322
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

